Abstract
Introduction
The prognostic value of total metabolic tumor volume (TMTV) at diagnosis in follicular lymphoma (FL) remains debated. Its impact was reported for patients treated mostly with Rituximab (R)-CHOP chemotherapy without R maintenance (FOLLCOLL study, Meignan et al, J Clin Oncol. 2016; 34(30):3618-3626) but manual TMTV assessment was not confirmed as prognostic in the GALLIUM trial where most patients had bendamustine and anti-CD20 antibody maintenance (Barrington et al, Blood 2018; 132(Suppl 1):2882). There is no data on the prognostic value of TMTV in chemo-free regimens such as the immunomodulatory combination of lenalidomide and rituximab (R2). Here, we investigated the prognostic value of baseline TMTV for FL patients with previously untreated advanced FL included in in the phase 3 RELEVANCE trial comparing R2vs R-chemo with both regimens followed by rituximab maintenance therapy (NCT01476787 and NCT01650701).
Methods
TMTV was computed on baseline PET/CT using the 41% SUVmax method. To separate high and low TMTV, we tested the previously published cut-off of 510cm3 (Meignan et al. J Clin Oncol. 2016; 34(30):3618-3626). Survival was estimated using Kaplan Meier curves. Analyses were performed on the total PET-evaluable population (n=406/1032) with 196 pts on the R2 arm and 210 pts on the R chemo arm, because baseline PET was not mandatory in this trial.
Results
Clinical baseline characteristics of PET evaluable patients were similar to the overall trial population for age, Ann Arbor stage, bone marrow infiltration, LDH, beta 2 microglobulin and FLIPI, and well balanced between the two treatment arms. After a median 6.5 years follow-up, 5y-PFS was 67.6%, 5y-OS was 93.5%, with no significant difference between the two arms.
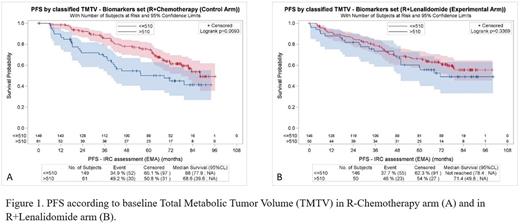
Median baseline TMTV was 284 cm3 in the whole cohort (Q1-Q3; 144-553) : 274 cm3 (129-526) in the R2 arm and 288 cm3 (148-562) in the R chemo arm (p=0.579). Overall, patients with TMTV > 510 cm3 had higher Ann Arbor stage (p<0.001), more extra nodal sites (p<0.001), more frequently elevated beta 2 microglobulin (p<0.001), higher FLIPI (p=0.002) and FLIPI2 scores (p<0.001). High TMTV was significantly associated with an inferior PFS (p=0.0121) and POD 24 (p=0.0014) but not OS (p=0.247). Patients with a high TMTV (n=111, 27%) had a 5-year PFS of 53.9% (CI 95% 43.6-63.1) vs 70.5% (CI 95% 64.7-75.6) for low TMTV patients. By contrast, bulk as measured by CT (either >6cm or >7cm) was not prognostic of PFS (p=0.978 and p=0.661 respectively). When testing FLIPI with TMTV both were independently associated with PFS (p=0.0119 and p=0.0379, respectively). When testing FLIPI2 with TMTV, only TMTV remained significant (p=0.2444 for FLIPI2, p=0.0285 for TMTV). The combination of FLIPI and TMTV stratified the overall population into 3 risk groups : patients with no risk factors had a 5y-PFS of 76.3% (n=162, 40% reference), declining to 63.4% for patients with 1 risk factor (n=180, 44%; HR=1.45 ;[1.01-2.06], p=0.042) and 47.1% (n=64, 16%; HR=2.15 [1.39-3.31], p<0.001) for patients with both. Although the interaction between TMTV and randomization arm was not statistically significant for PFS (p=0.31), the prognostic impact of TMTV appeared different between the two arms: a high TMTV was associated with a lower PFS in the R chemo arm (Figure 1 A: p=0.0093) whereas it was not significant in the R2 arm (Figure 1B: p=0.3369).
Conclusion: Baseline TMTV was a strong prognostic factor independent of FLIPI or FLIPI2 status, in advanced FL patients treated with first-line immunochemotherapy followed by antibody maintenance but not R2.
Disclosures
Trotman:Roche: Research Funding; Beigene: Research Funding; Takeda: Research Funding; Cellectar: Research Funding; BMS: Research Funding; PCYC: Research Funding; Janssen: Other: clinical trials. Feugier:AstraZeneca, Janssen, Abbvie, Beigene, Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Congress Invitations. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Bachy:Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Flinn:MorphoSys: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Gilead Sciences: Research Funding; Unum Therapeutics: Research Funding; Agios: Research Funding; ArQule: Research Funding; Acerta Pharma: Research Funding; Xencor: Consultancy; Constellation Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Celgene: Research Funding; Iksuda Therapeutics: Consultancy; BeiGene: Consultancy, Research Funding; Hutchison MediPharma: Consultancy; Verastem: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Servier Pharmaceuticals: Consultancy; Roche: Consultancy, Research Funding; Myeloid Therapeutics: Research Funding; Trillium Therapeutics: Research Funding; Secura Bio: Consultancy; Biopath: Research Funding; Genmab: Consultancy; Bristol Myers Squibb: Research Funding; Genentech: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Research Funding; IGM Biosciences: Research Funding; Forty Seven: Research Funding; Pfizer: Research Funding; Forma Therapeutics: Research Funding; Portola Pharmaceuticals: Research Funding; Curis: Research Funding; Century Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Incyte: Research Funding; Merck: Research Funding; Infinity Pharmaceuticals: Research Funding; Loxo@Lilly: Research Funding; CALIBR: Research Funding; CALGB: Research Funding; Abbvie: Consultancy, Research Funding; 2seventy bio: Research Funding; Triphase Research & Development Corp: Research Funding; City of Hope National Medical Center: Research Funding; Epizyme: Research Funding; CTI Biopharma: Research Funding; Tessa Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Millenium Pharmaceuticals: Research Funding; TCR2 Therapeutics: Research Funding. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Bartlett:Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; Washington University School of Medicine: Current Employment; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tilly:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Casasnovas:Roche, Takeda, BMS, MSD, Gilead/Kite, Janssen, ADC Therapeutics, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche, Gilead, Takeda: Research Funding; Roche, Takeda, Merck, BMS, Gilead/Kite, Abbvie, ADC therapeutics, INCYTE, AstraZeneca: Honoraria. Meignan:Roche: Other: supported the organisation of a virtual meeting; Novartis: Other: supported the organisation of a virtual meeting. Morschhauser:F. Hoffmann-La Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Servier: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal